About Us
Yuhuan Zhengri Technology Co., Ltd.
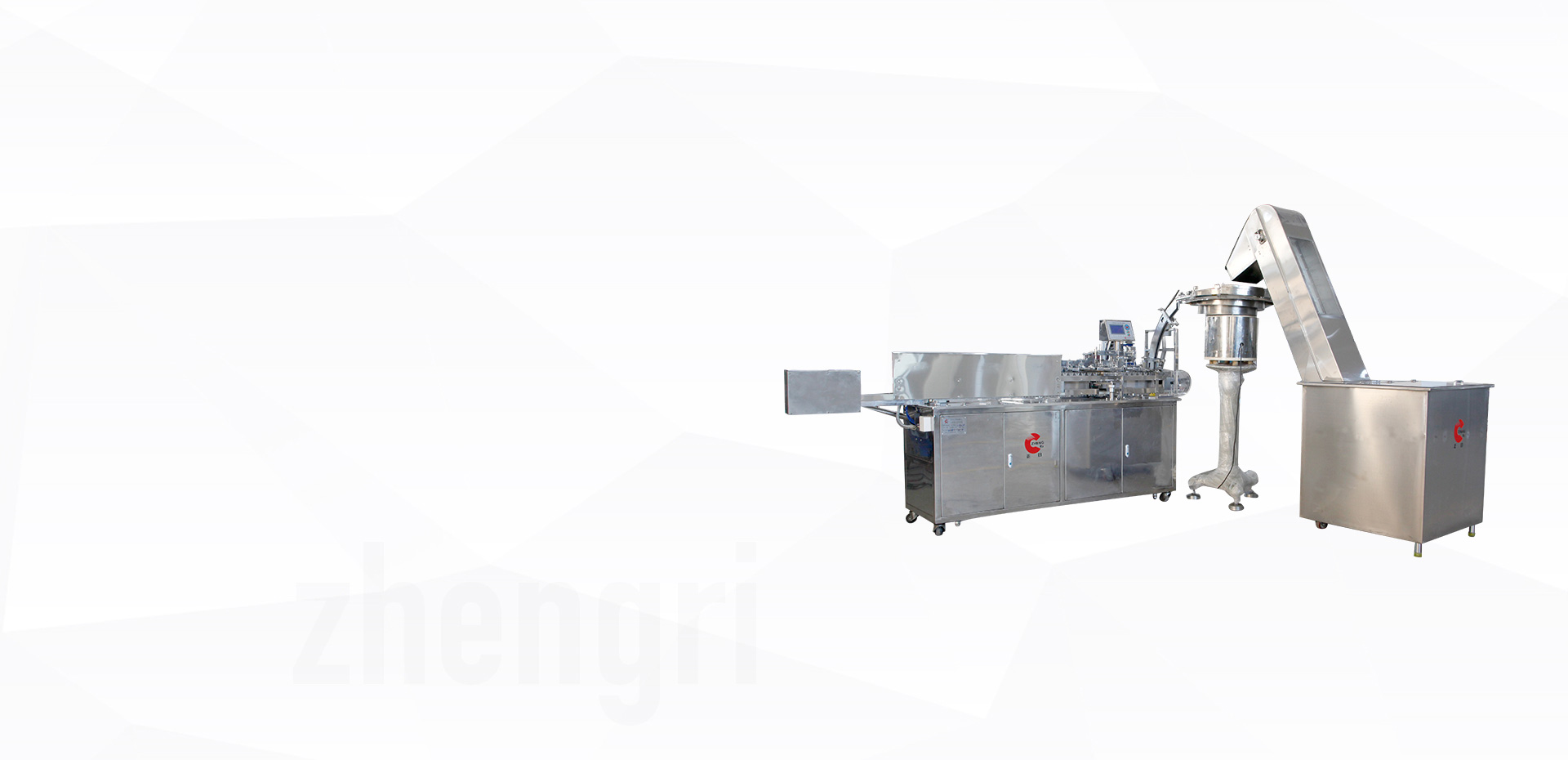
leading manufacturer of medical machine in China
. is located ateast sea of zhejiang beautiful city--qinggang scientific & technologic park. YUHUAN CIUNTY, our company is between Ning Bo and Wen Zhou opening harbours.The communication is very convenient. Our company has passed ISO9001 :2000 international quality certificate, long term specialized manufacturing and develop all kinds of injection all automatic assembly machine, injection, screen printer, pen tube……

 Chinese
Chinese English
English